基于GQDs的荧光猝灭检测抗坏血酸的新方法(4)
时间:2017-06-20 来源:发光学报 作者:赵丽敏,陈丽妮,赵书林 本文字数:8943字 3. 7 特异性考察。
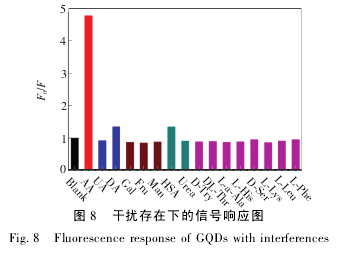
为了考察所建立方法的特异性,实验选用几种糖类以及 HSA、尿素( Urea) 和几种氨基酸作为对照样品进行分析。GQDs 的浓度为 0. 14 mg/mL,AA 的浓度为 7. 5 × 10- 5mol / L,其他作为干扰物质的浓度均为 7. 5 × 10- 5mol / L.实验结果如图 8 所示。从图中可以看出,只有 AA 的加入能引起信号的明显变化,其他物质均不存在干扰,特别是 DA、UA 这两种与 AA 有相似电化学性质的物质,也不存在干扰。因为在本实验中,AA 与GQDs 的反应介质为 pH = 4. 5 的磷酸盐缓冲液,在这个 pH 值下,DA、UA 均不干扰 AA 的测定。
 3. 8 实际样品分析。
3. 8 实际样品分析。
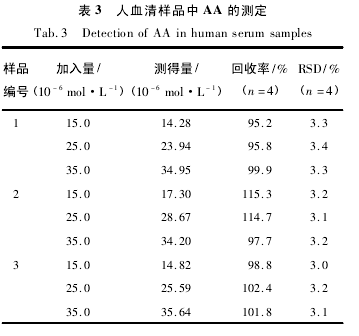
为了考察本研究所建立的方法是否可用于实际样品的检测,实验对人血清样品进行了分析。从医院取的血清样品按照 2. 2 节方法进行处理,在稀释 50 倍后的血清中进行加标实验,实验结果如表 3 所示。从表中可以看到,人血清中 AA 的加标回收率在 95. 2% ~115. 3%范围内。
 4 结 论。
4 结 论。
建立了一种基于 GQDs 荧光猝灭检测 AA的新方法,该方法操作简便,灵敏度高,检出限低至 1. 0 ×10- 6mol / L,特异性强,一些糖类和氨基酸的存在均不干扰 AA 的测定,甚至是与 AA有相似电化学性质的 DA、UA 的存在对 AA 的检测也没有影响。本实验用到的所有原料都价廉易得且不需要任何修饰过程,GQDs 相对于其他量子点的合成更简单而且具有低毒性,有望应用于生物体内生物活性物质的检测。
参 考 文 献:
[1]PONOMARENKO L A,SCHEDIN F,KATSNELSON M I,et al. . Chaotic dirac billiard in graphene quantum dots [J].Science,2008,320( 5874) : 356-358.
[2]PAN D Y,ZHANG J C,LI Z,et al. . Hydrothermal route for cutting graphene sheets into blue-luminescent graphenequantum dots [J]. Adv. Mater. ,2010,22( 6) : 734-738.
[3]CHENG H H,ZHAO Y,FAN Y Q,et al. . Graphene-quantum-dot assembled nanotubes: a new platform for efficient Ra-man enhancement [J]. ACS Nano,2012,6( 3) : 2237-2244.
[4]DONG Y Q,CHEN C Q,ZHENG X T,et al. . One-step and high yield simultaneous preparation of single-and multi-layergraphene quantum dots from CX-72 carbon black [J]. J. Mater. Chem. ,2012,22( 18) : 8764-8766.
[5]SHEN J H,ZHU Y H,YANG X L,et al. . Graphene quantum dots: emergent nanolights for bioimaging,sensors,cataly-sis and photovoltaic devices [J]. Chem. Commun. ,2012,48( 31) : 3686-3699.
[6]LU J,YEO P S E,GAN C K,et al. . Transforming C60molecules into graphene quantum dots [J]. Nat. Nanotechnol. ,2011,6( 4) : 247-252.
[7]LIU R L,WU D Q,FENG X L,et al. . Bottom-up fabrication of photoluminescent graphene quantum dots with uniformmorphology [J]. J. Am. Chem. Soc. ,2011,133( 39) : 15221-15223.
[8]NIU Z Q,CHEN J,HNG H H,et al. . A leavening strategy to prepare reduced graphene oxide foams[J]. Adv. Mater. ,2012,24( 30) : 4144-4150.
[9]DONG Y Q,LI G L,ZHOU N N,et al. . Graphene quantum dot as a green and facile sensor for free chlorine in drinkingwater [J]. Anal. Chem. ,2012,84( 19) : 8378-8382.
[10]BAI J M,ZHANG L,LIANG R P,et al. . Graphene quantum dots combined with europium ions as photoluminescentprobes for phosphate sensing [J]. Chem. Eur. J. ,2013,19( 12) : 3822-3826.
[11]LIU J J,ZHANG X L,CONG Z X,et al. . Glutathione-functionalized graphene quantum dots as selective fluorescentprobes for phosphate-containing metabolites [J]. Nanoscale,2013,5( 5) : 1810-1815.
[12]WANG D,WANG L,DONG X Y,et al. . Chemically tailoring graphene oxides into fluorescent nanosheets for Fe3 +iondetection [J]. Carbon,2012,50( 6) : 2147-2154.
[13]ZHANG Y,WU C Y,ZHOU X J,et al. . Graphene quantum dots/gold electrode and its application in living cell H2O2detection [J]. Nanoscale,2013,5( 5) : 1816-1819.
[14]LI Y H,ZHANG L,HUANG J,et al. Fluorescent graphene quantum dots with a boronic acid appended bipyridinium saltto sense monosaccharides in aqueous solution [J]. Chem. Commun. ,2013,49( 45) : 5180-5182.
[15]RAZMI H,MOHAMMAD-REZAEI R. Graphene quantum dots as a new substrate for immobilization and direct electro-chemistry of glucose oxidase: application to sensitive glucose determination [J]. Biosens. Bioelectron. ,2013,41:498-504.
[16]ZHAO H M,CHANG Y Y,LIU M,et al. . A universal immunosensing strategy based on regulation of the interaction be-tween graphene and graphene quantum dots [J]. Chem. Commun. ,2013,49( 3) : 234-236.
[17]RAN X,SUN H J,PU F,et al. . Ag nanoparticle-decorated graphene quantum dots for label-free,rapid and sensitive de-tection of Ag+and biothiols [J]. Chem. Commun. ,2013,49( 11) : 1079-1081.
[18]ZHOU L,LIN Y H,HUANG Z Z,et al. . Carbon nanodots as fluorescence probes for rapid,sensitive,and label-free de-tection of Hg2 +and biothiols in complex matrices [J]. Chem. Commun. ,2012,48( 8) : 1147-1149.
[19]ZHAO J,CHEN G F,ZHU L,et al. . Graphene quantum dots-based platform for the fabrication of electrochemical biosen-sors [J]. Electrochem. Commun. ,2011,13( 1) : 31-33.
[20]LI X,ZHU S J,XU B,et al. Self-assembled graphene quantum dots induced by cytochrome C: a novel biosensor for tryp-sin with remarkable fluorescence enhancement [J]. Nanoscale,2013,5( 17) : 7776-7779.
[21]JING Y J,ZHU Y H,YANG X L,et al. . Ultrasound-triggered smart drug release from multifunctional core-shell capsulesone-step fabricated by coaxial electrospray method [J]. Langmuir,2011,27( 3) : 1175-1180.
[22]PENG J,GAO W,GUPTA B K,et al. . Graphene quantum dots derived from carbon fibers[J]. Nano Lett. ,2012,12( 2) :844-849.
[23]ZHU S J,ZHANG J H,QIAO C Y,et al. . Strongly green-photoluminescent graphene quantum dots for bioimaging appli-cations [J]. Chem. Commun. ,2011,47( 24) : 6858-6860.
[24]ZHANG M,BAI L L,SHANG W H,et al. . Facile synthesis of water-soluble,highly fluorescent graphene quantum dotsas a robust biological label for stem cells [J]. J. Mater. Chem. ,2012,22( 15) : 7461-7467.
[25]XIAO L F,CHEN J,CHA C S. Elimination of the interference of ascorbic acid in the amperometric detection of biomole-cules in body fluid samples and the simple detection of uric acid in human serum and urine by using the powder microelec-trode technique [J]. J. Electroanal. Chem. ,2000,495( 1) : 27-35.
[26]SOOD S P,SARTORI L E,WITTMER D P,et al. . High-pressure liquid chromatographic determination of ascorbic acidin selected foods and multivitamin products [J]. Anal. Chem. ,1976,48( 6) : 796-798.
[27]WANG Z H,LIU J,LIANG Q L,et al. . Carbon nanotube-modified electrodes for the simultaneous determination of dopa-mine and ascorbic acid [J]. Analyst,2002,127( 5) : 653-658.
[28]CHEN H,LI R B,LIN L,et al. . Determination of L-ascorbic acid in human serum by chemiluminescence based on hy-drogen peroxide-sodium hydrogen carbonate-CdSe / CdS quantum dots system [J]. Talanta,2010,81( 4-5) : 1688-1696.
[29]SHAKYA R,NAVARRE D A. Rapid screening of ascorbic acid,glycoalkaloids,and phenolics in potato using high-per-formance liquid chromatography [J]. J. Agric. Food Chem. ,2006,54( 15) : 5253-5260.
[30]BOSSI A,PILETSKY S A,PILETSKA E V,et al. . An assay for ascorbic acid based on polyaniline-coated microplates[J]. Anal. Chem. ,2000,72( 18) :4296-4300.
[31]CASTELLETTI L,PILETSKY S A,TURNER A P F,et al. . Development of an integrated capillary electrophoresis/sensorfor L-ascorbic acid detection [J]. Electrophoresis,2002,23( 2) : 209-214.
[32]WANG X X,WU P,HOU X D,et al. An ascorbic acid sensor based on protein-modified Au nanoclusters[J]. Analyst,2013,138( 1) : 229-233.
[33]DONG Y Q,SHAO J W,CHEN C Q,et al. . Blue luminescent graphene quantum dots and graphene oxide prepared bytuning the carbonization degree of citric acid [J]. Carbon,2012,50( 12) : 4738-4743.
[34]ZHAO J J,ZHAO L M,LAN C Q,et al. . Graphene quantum dots as effective probes for label-free fluorescence detectionof dopamine [J]. Sens. Actuators B,2016,223: 246-251.
[35]ZHANG J L,YANG H J,SHEN G X,et al. . Reduction of graphene oxide via L-ascorbic acid[J]. Chem. Commun. ,2010,46( 7) : 1112-1114.
[36]BAKER S N,BAKER G A. Luminescent carbon nanodots: emergent nanolights[J]. Angew. Chem. Int. Ed. ,2010,49( 38) :6726-6744.
[37]MITRA S,CHANDRA S,PATHAN S H,et al. . Room temperature and solvothermal green synthesis of self passivatedcarbon quantum dots [J]. RSC Adv. ,2013,3( 10) : 3189-3193.
[38]BALZANI V,CREDI A,VENTURI M. Molecular Devices and Machines: Concepts and Perspectives for The Nanoworld[M]. 2nd ed. New York: Wiley,2009:33.
[39]LIU M L,CHEN Q,LAI C L,et al. . A double signal amplification platform for ultrasensitive and simultaneous detectionof ascorbic acid,dopamine,uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@ Au nanoparticles with graphene sheet [J]. Biosens. Bioelectron. ,2013,48: 75-81.
[40]HOU T,GAI P P,SONG M M,et al. . Synthesis of a three-layered SiO2@ Au nanoparticle@ polyaniline nanocompositeand its application in simultaneous electrochemical detection of uric acid and ascorbic acid [J]. J. Mater. Chem. B,2016,4( 13) : 2314-2321.
[41]YANG Y J. One-pot synthesis of reduced graphene oxide/zinc sulfide nanocomposite at room temperature for simultaneousdetermination of ascorbic acid,dopamine and uric acid [J]. Sens. Actuators B,2015,221: 750-759.
[42]ZHAO D Y,YU G L,TIAN K L,et al. . A highly sensitive and stable electrochemical sensor for simultaneous detectiontowards ascorbic acid,dopamine,and uric acid based on the hierarchical nanoporous PtTi alloy [J]. Biosens. Bioelec-tron. ,2016,82: 119-126.
[43]ZHANG X,ZHANG Y C,MA L X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivi-ty for simultaneous electrochemical detection of ascorbic acid,dopamine and uric acid [J]. Sens. Actuators B,2016,227: 488-496.
[44]MI C C,WANG T T,ZENG P,et al. . Determination of ascorbic acid via luminescence quenching of LaF3∶ Ce,Tb nanop-articles synthesized through a microwave-assisted solvothermal method [J]. Anal. Methods,2013,5( 6) : 1463-1468.
[45]MA Q,LI Y,LIN Z H,et al. . A novel ascorbic acid sensor based on the Fe3 +/ Fe2 +modulated photoluminescence ofCdTe quantum dots@ SiO2nanobeads [J]. Nanoscale,2013,5( 20) : 9726-9731.
为了考察所建立方法的特异性,实验选用几种糖类以及 HSA、尿素( Urea) 和几种氨基酸作为对照样品进行分析。GQDs 的浓度为 0. 14 mg/mL,AA 的浓度为 7. 5 × 10- 5mol / L,其他作为干扰物质的浓度均为 7. 5 × 10- 5mol / L.实验结果如图 8 所示。从图中可以看出,只有 AA 的加入能引起信号的明显变化,其他物质均不存在干扰,特别是 DA、UA 这两种与 AA 有相似电化学性质的物质,也不存在干扰。因为在本实验中,AA 与GQDs 的反应介质为 pH = 4. 5 的磷酸盐缓冲液,在这个 pH 值下,DA、UA 均不干扰 AA 的测定。

为了考察本研究所建立的方法是否可用于实际样品的检测,实验对人血清样品进行了分析。从医院取的血清样品按照 2. 2 节方法进行处理,在稀释 50 倍后的血清中进行加标实验,实验结果如表 3 所示。从表中可以看到,人血清中 AA 的加标回收率在 95. 2% ~115. 3%范围内。

建立了一种基于 GQDs 荧光猝灭检测 AA的新方法,该方法操作简便,灵敏度高,检出限低至 1. 0 ×10- 6mol / L,特异性强,一些糖类和氨基酸的存在均不干扰 AA 的测定,甚至是与 AA有相似电化学性质的 DA、UA 的存在对 AA 的检测也没有影响。本实验用到的所有原料都价廉易得且不需要任何修饰过程,GQDs 相对于其他量子点的合成更简单而且具有低毒性,有望应用于生物体内生物活性物质的检测。
参 考 文 献:
[1]PONOMARENKO L A,SCHEDIN F,KATSNELSON M I,et al. . Chaotic dirac billiard in graphene quantum dots [J].Science,2008,320( 5874) : 356-358.
[2]PAN D Y,ZHANG J C,LI Z,et al. . Hydrothermal route for cutting graphene sheets into blue-luminescent graphenequantum dots [J]. Adv. Mater. ,2010,22( 6) : 734-738.
[3]CHENG H H,ZHAO Y,FAN Y Q,et al. . Graphene-quantum-dot assembled nanotubes: a new platform for efficient Ra-man enhancement [J]. ACS Nano,2012,6( 3) : 2237-2244.
[4]DONG Y Q,CHEN C Q,ZHENG X T,et al. . One-step and high yield simultaneous preparation of single-and multi-layergraphene quantum dots from CX-72 carbon black [J]. J. Mater. Chem. ,2012,22( 18) : 8764-8766.
[5]SHEN J H,ZHU Y H,YANG X L,et al. . Graphene quantum dots: emergent nanolights for bioimaging,sensors,cataly-sis and photovoltaic devices [J]. Chem. Commun. ,2012,48( 31) : 3686-3699.
[6]LU J,YEO P S E,GAN C K,et al. . Transforming C60molecules into graphene quantum dots [J]. Nat. Nanotechnol. ,2011,6( 4) : 247-252.
[7]LIU R L,WU D Q,FENG X L,et al. . Bottom-up fabrication of photoluminescent graphene quantum dots with uniformmorphology [J]. J. Am. Chem. Soc. ,2011,133( 39) : 15221-15223.
[8]NIU Z Q,CHEN J,HNG H H,et al. . A leavening strategy to prepare reduced graphene oxide foams[J]. Adv. Mater. ,2012,24( 30) : 4144-4150.
[9]DONG Y Q,LI G L,ZHOU N N,et al. . Graphene quantum dot as a green and facile sensor for free chlorine in drinkingwater [J]. Anal. Chem. ,2012,84( 19) : 8378-8382.
[10]BAI J M,ZHANG L,LIANG R P,et al. . Graphene quantum dots combined with europium ions as photoluminescentprobes for phosphate sensing [J]. Chem. Eur. J. ,2013,19( 12) : 3822-3826.
[11]LIU J J,ZHANG X L,CONG Z X,et al. . Glutathione-functionalized graphene quantum dots as selective fluorescentprobes for phosphate-containing metabolites [J]. Nanoscale,2013,5( 5) : 1810-1815.
[12]WANG D,WANG L,DONG X Y,et al. . Chemically tailoring graphene oxides into fluorescent nanosheets for Fe3 +iondetection [J]. Carbon,2012,50( 6) : 2147-2154.
[13]ZHANG Y,WU C Y,ZHOU X J,et al. . Graphene quantum dots/gold electrode and its application in living cell H2O2detection [J]. Nanoscale,2013,5( 5) : 1816-1819.
[14]LI Y H,ZHANG L,HUANG J,et al. Fluorescent graphene quantum dots with a boronic acid appended bipyridinium saltto sense monosaccharides in aqueous solution [J]. Chem. Commun. ,2013,49( 45) : 5180-5182.
[15]RAZMI H,MOHAMMAD-REZAEI R. Graphene quantum dots as a new substrate for immobilization and direct electro-chemistry of glucose oxidase: application to sensitive glucose determination [J]. Biosens. Bioelectron. ,2013,41:498-504.
[16]ZHAO H M,CHANG Y Y,LIU M,et al. . A universal immunosensing strategy based on regulation of the interaction be-tween graphene and graphene quantum dots [J]. Chem. Commun. ,2013,49( 3) : 234-236.
[17]RAN X,SUN H J,PU F,et al. . Ag nanoparticle-decorated graphene quantum dots for label-free,rapid and sensitive de-tection of Ag+and biothiols [J]. Chem. Commun. ,2013,49( 11) : 1079-1081.
[18]ZHOU L,LIN Y H,HUANG Z Z,et al. . Carbon nanodots as fluorescence probes for rapid,sensitive,and label-free de-tection of Hg2 +and biothiols in complex matrices [J]. Chem. Commun. ,2012,48( 8) : 1147-1149.
[19]ZHAO J,CHEN G F,ZHU L,et al. . Graphene quantum dots-based platform for the fabrication of electrochemical biosen-sors [J]. Electrochem. Commun. ,2011,13( 1) : 31-33.
[20]LI X,ZHU S J,XU B,et al. Self-assembled graphene quantum dots induced by cytochrome C: a novel biosensor for tryp-sin with remarkable fluorescence enhancement [J]. Nanoscale,2013,5( 17) : 7776-7779.
[21]JING Y J,ZHU Y H,YANG X L,et al. . Ultrasound-triggered smart drug release from multifunctional core-shell capsulesone-step fabricated by coaxial electrospray method [J]. Langmuir,2011,27( 3) : 1175-1180.
[22]PENG J,GAO W,GUPTA B K,et al. . Graphene quantum dots derived from carbon fibers[J]. Nano Lett. ,2012,12( 2) :844-849.
[23]ZHU S J,ZHANG J H,QIAO C Y,et al. . Strongly green-photoluminescent graphene quantum dots for bioimaging appli-cations [J]. Chem. Commun. ,2011,47( 24) : 6858-6860.
[24]ZHANG M,BAI L L,SHANG W H,et al. . Facile synthesis of water-soluble,highly fluorescent graphene quantum dotsas a robust biological label for stem cells [J]. J. Mater. Chem. ,2012,22( 15) : 7461-7467.
[25]XIAO L F,CHEN J,CHA C S. Elimination of the interference of ascorbic acid in the amperometric detection of biomole-cules in body fluid samples and the simple detection of uric acid in human serum and urine by using the powder microelec-trode technique [J]. J. Electroanal. Chem. ,2000,495( 1) : 27-35.
[26]SOOD S P,SARTORI L E,WITTMER D P,et al. . High-pressure liquid chromatographic determination of ascorbic acidin selected foods and multivitamin products [J]. Anal. Chem. ,1976,48( 6) : 796-798.
[27]WANG Z H,LIU J,LIANG Q L,et al. . Carbon nanotube-modified electrodes for the simultaneous determination of dopa-mine and ascorbic acid [J]. Analyst,2002,127( 5) : 653-658.
[28]CHEN H,LI R B,LIN L,et al. . Determination of L-ascorbic acid in human serum by chemiluminescence based on hy-drogen peroxide-sodium hydrogen carbonate-CdSe / CdS quantum dots system [J]. Talanta,2010,81( 4-5) : 1688-1696.
[29]SHAKYA R,NAVARRE D A. Rapid screening of ascorbic acid,glycoalkaloids,and phenolics in potato using high-per-formance liquid chromatography [J]. J. Agric. Food Chem. ,2006,54( 15) : 5253-5260.
[30]BOSSI A,PILETSKY S A,PILETSKA E V,et al. . An assay for ascorbic acid based on polyaniline-coated microplates[J]. Anal. Chem. ,2000,72( 18) :4296-4300.
[31]CASTELLETTI L,PILETSKY S A,TURNER A P F,et al. . Development of an integrated capillary electrophoresis/sensorfor L-ascorbic acid detection [J]. Electrophoresis,2002,23( 2) : 209-214.
[32]WANG X X,WU P,HOU X D,et al. An ascorbic acid sensor based on protein-modified Au nanoclusters[J]. Analyst,2013,138( 1) : 229-233.
[33]DONG Y Q,SHAO J W,CHEN C Q,et al. . Blue luminescent graphene quantum dots and graphene oxide prepared bytuning the carbonization degree of citric acid [J]. Carbon,2012,50( 12) : 4738-4743.
[34]ZHAO J J,ZHAO L M,LAN C Q,et al. . Graphene quantum dots as effective probes for label-free fluorescence detectionof dopamine [J]. Sens. Actuators B,2016,223: 246-251.
[35]ZHANG J L,YANG H J,SHEN G X,et al. . Reduction of graphene oxide via L-ascorbic acid[J]. Chem. Commun. ,2010,46( 7) : 1112-1114.
[36]BAKER S N,BAKER G A. Luminescent carbon nanodots: emergent nanolights[J]. Angew. Chem. Int. Ed. ,2010,49( 38) :6726-6744.
[37]MITRA S,CHANDRA S,PATHAN S H,et al. . Room temperature and solvothermal green synthesis of self passivatedcarbon quantum dots [J]. RSC Adv. ,2013,3( 10) : 3189-3193.
[38]BALZANI V,CREDI A,VENTURI M. Molecular Devices and Machines: Concepts and Perspectives for The Nanoworld[M]. 2nd ed. New York: Wiley,2009:33.
[39]LIU M L,CHEN Q,LAI C L,et al. . A double signal amplification platform for ultrasensitive and simultaneous detectionof ascorbic acid,dopamine,uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@ Au nanoparticles with graphene sheet [J]. Biosens. Bioelectron. ,2013,48: 75-81.
[40]HOU T,GAI P P,SONG M M,et al. . Synthesis of a three-layered SiO2@ Au nanoparticle@ polyaniline nanocompositeand its application in simultaneous electrochemical detection of uric acid and ascorbic acid [J]. J. Mater. Chem. B,2016,4( 13) : 2314-2321.
[41]YANG Y J. One-pot synthesis of reduced graphene oxide/zinc sulfide nanocomposite at room temperature for simultaneousdetermination of ascorbic acid,dopamine and uric acid [J]. Sens. Actuators B,2015,221: 750-759.
[42]ZHAO D Y,YU G L,TIAN K L,et al. . A highly sensitive and stable electrochemical sensor for simultaneous detectiontowards ascorbic acid,dopamine,and uric acid based on the hierarchical nanoporous PtTi alloy [J]. Biosens. Bioelec-tron. ,2016,82: 119-126.
[43]ZHANG X,ZHANG Y C,MA L X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivi-ty for simultaneous electrochemical detection of ascorbic acid,dopamine and uric acid [J]. Sens. Actuators B,2016,227: 488-496.
[44]MI C C,WANG T T,ZENG P,et al. . Determination of ascorbic acid via luminescence quenching of LaF3∶ Ce,Tb nanop-articles synthesized through a microwave-assisted solvothermal method [J]. Anal. Methods,2013,5( 6) : 1463-1468.
[45]MA Q,LI Y,LIN Z H,et al. . A novel ascorbic acid sensor based on the Fe3 +/ Fe2 +modulated photoluminescence ofCdTe quantum dots@ SiO2nanobeads [J]. Nanoscale,2013,5( 20) : 9726-9731.
- 相关内容推荐
相近分类:
推荐阅读





