����ժ Ҫ��������CeO2���ھ�����������ܶ����㷺Ӧ���ڴ�����������ϡ��ȵ���Ϻ����崫����������;����������ܲ������䱾����������ѧ�����й�, ��ȡ���ڲ��ϵ�������С����˿ɿغϳɲ�ͬ��������ò���������CeO2���о������ܵ��Ⱦ���������Ce (NO3) 3·6H2O��NaOHΪԭ��, ͨ��ˮ�ȷ��ɿغϳ�������ΧΪ19.659.8 nm����������CeO2, ���о��˲�ͬʵ����������������Ӱ�졣�о��������, ˮ���¶ȡ�NaOH��Ũ�Ⱥͱ�����Լ���������Ӱ����������Ҫ����, ����ˮ���¶ȵ�����, ����������;����NaOHŨ�ȵ�����, ����CeO2�������ȳ����������, ��NaOH��Ũ�ȹ���ʱ, ����CeO2�������ֳʼ�С������;��ϵ�м��벻ͬ�ı�����Լ�������������ͬ��Ӱ��:���Ҷ���-2000����������ʹ����CeO2���������Լ�С, �����Ҷ���-400������෴�Ľ����
�����ؼ��ʣ�������CeO2; �ϳ�; ˮ�ȷ�; ����; Ӱ������;
����Abstract����Nano-CeO2 has been widely applied on catalysts, hydrogen storage materials, thermoelectric materials and gas sensors due to its plentiful excellent properties which not only depend on the physical and chemical properties but also on the particle size and the morphology.Therefore, the synthesis of nano-CeO2 with proper particle size and regular morphology is a prerequisite for investigating its properties.Using Ce (NO3) 3·6H2O and NaOH as raw materials, the cubic nano-CeO2 with different average diameters (19. 6 ~ 59. 8 nm) was synthesized by hydrothermal method, and the influence regularities of different experimental conditions on the particle size of nano-CeO2 were investigated. The results indicated that the hydrothermal temperature, the concentration of NaOH and the variety of surfactants were the main effect factors on the particle size. The particle size increased with increase of the hydrothermal temperature.With the increase of the concentration of NaOH, the particle size of nano-CeO2 increased first.When the concentration of NaOH was too high, the particle size decreased.Adding different surfactants in the reaction fluid had different effects on the particle size: adding polyethylene glycol-2000 and sodium citrate could reduce the particle size significantly, while adding polyethylene glycol-400 was the opposite.
����Keyword����nano-CeO2; synthesis; hydrothermal method; particle size; influencing factors;
����Ce O2��Ϊһ�ֵ��͵�ϡ��������, ��������Ĺ⡢�硢�ŵ�����[1];����Ce O2���˾߱�����������, ���������ײ��϶��е�����, ��ߴ�С���ȱ������, ʹ��㷺Ӧ���ڹ�����������ⲣ����������ϡ�ȼ�ϵ�ء����崫�����������մɵ�����[2,3,4,5]��Ȼ��, ��ͬ����������Ce O2�������ܺ�Ӧ�������ŵ�Ӱ�졣�����[6]��������Ce O2������ԽС, ��CO�Ĵ�����Խ��;��[7]�о���������Ce O2����������������Ч�������ŵ�Ӱ��;��ͬ������������Ce O2������������Ҳ����ͬ, Ce O2������ԽС, ������ȱ��Խ��, ����������Ҳ��Խ��[8]�����, �ɿغϳɲ�ͬ��������ò���������Ce O2���о������ʵ��Ⱦ�������
����Ŀǰ, ����Ce O2�ĺϳɷ�����Ҫ����������[9]���ܽ�-������[10]����Һ��[11]��ˮ�ȷ�/�ܼ��ȷ�[12,13]�ȡ�Chu��[14]��Ce (NO3) 3������Ϊԭ��, ���ó������ɹ��ϳɳ�����Ϊ200~300nm����������Ce O2, ������ķ�ɢ�Բʹ����Һ���ϳ�����Ce O2�����������֮���ž۵�����, ������ʵ������˴��ģ��������ˮ�ȷ����в�����, ������ɢ�Ժ�, �����ֲ����ȵ��ŵ�, ���Ҵ˷����������ܺĵ�[15]���������[16]����ˮ�ȷ���170���Ʊ��˾�����������ò��Ce O2������, ������������ò����, ���ǿ���������ƫ��, ��δ̽��ʵ��������������Ӱ����ɡ�����ͨ��ˮ�ȷ�, ��Ce (NO3) 3·6H2O��Na OHΪԭ�Ϻϳ���������Ce O2, ̽����ˮ���¶ȡ�Na OH��Ũ�ȡ�������Լ�������������¶ȶ�����Ce O2������Ӱ�����, �Ӷ�ʵ�ֲ�ͬ������������Ce O2�Ŀɿغϳɡ�
����1�� ʵ�鲿��
����1.1�� ��Ҫ�������Լ�
����6000��X-���������� (�ձ�����˾) ;JSM-2010����������� (�ձ�������ʽ����) ;BS224S�͵�����ƽ (����������˹����ϵͳ����˾) ;DF-101S�ͼ���ʽ���´��������� (�������軪�����������ι�˾) ;��λ�۱�ˮ�ȷ�Ӧ�� (�Ϻ�ӣ��ʵ����������˾) ��
���������� (������, ���⸴��ϸ��������˾) ;Na OH (������, ������Լ���) ;�������� (������, ���紬��ѧ�Լ�����˾) ;���Ҷ���-2000 (��ѧ��, ��ҩ���Ż�ѧ�Լ�����˾) ;���Ҷ���-400 (��ѧ��, ������ѧ�Լ���) ��
����1.2�� ��Ʒ�Ʊ�
������3.47 g Ce (NO3) 3·6H2O�ܽ20 m L����ˮ��;�ٽ�����μ��뵽140 m L (10.68 mol/L) Na OH��Һ��, ������Һ�м���һ�����ı�����Լ�������30 min��, �������õ��Ļ��Һת����ˮ�ȷ�Ӧ����, ������뵽100��ĺ����з�Ӧ24 h;��������ķ���, �ֱ���ȥ����ˮ���Ҵ�ϴ������, 80�����, �����500��������2 h, ���õ�����Ce O2������
����1.3�� ����
�����ֱ���X-���������Ǻ���羵�Բ���ľ��ͺ���ò���б���, ������Ʒ����羵ͼ, ����Nano Measurer�����õ�����Ce O2��ƽ��������
����2�� ���������
����2.1�� ˮ���¶ȶ�������Ӱ��
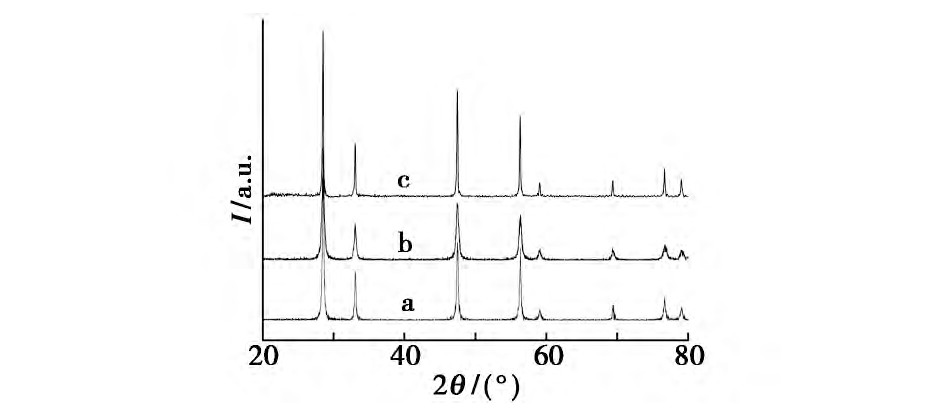
����������Ʒ���Ʊ�����, ���Һ�в����������Լ�, ���ı�ˮ���¶�̽�����������Ӱ��, �����Һ�ֱ���100��120��140��160��180����ˮ�ȷ�Ӧ24 h��ͼ1Ϊ��ͬˮ���¶��ºϳɵ�����Ce O2��XRDͼ���������, ����ˮ���¶ȵ�����, �ϳɵ�����Ce O2��XRD�����ǿ�����ű�ǿ, ���������խ, ��������ˮ���¶ȵ�����, ���ϳɵ���Ʒ�ᾧ�ȱ�á�������ˮ���¶�Ϊ180��ʱ���ϳɵ�����Ce O2�������������нϸߵ�ǿ��, �������ϳɵ���Ʒ�ᾧ�ȽϺá�
����ͼ1 ��ͬˮ���¶Ⱥϳɵ�����Ce O2��XRDͼFig.1 XRD Patterns of nano-Ce O2crystallites synthesized at different hydrothermal temperatures��
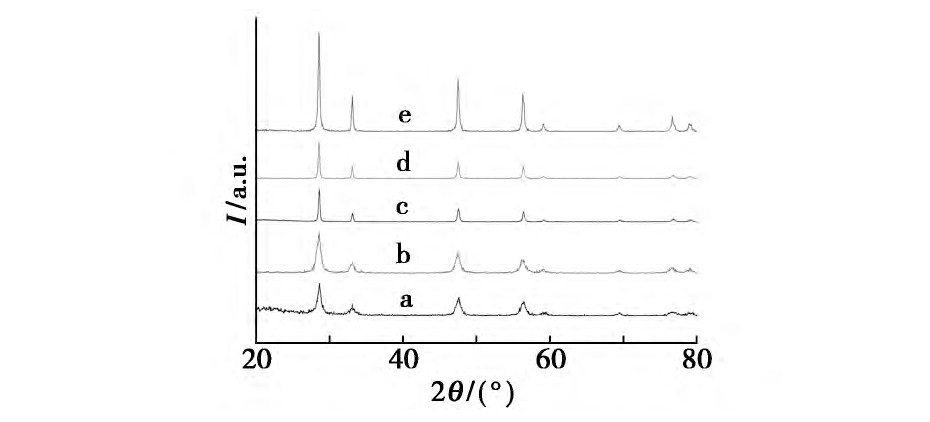
����a.100��;b.120��;c.140��;d.160��;e.180��
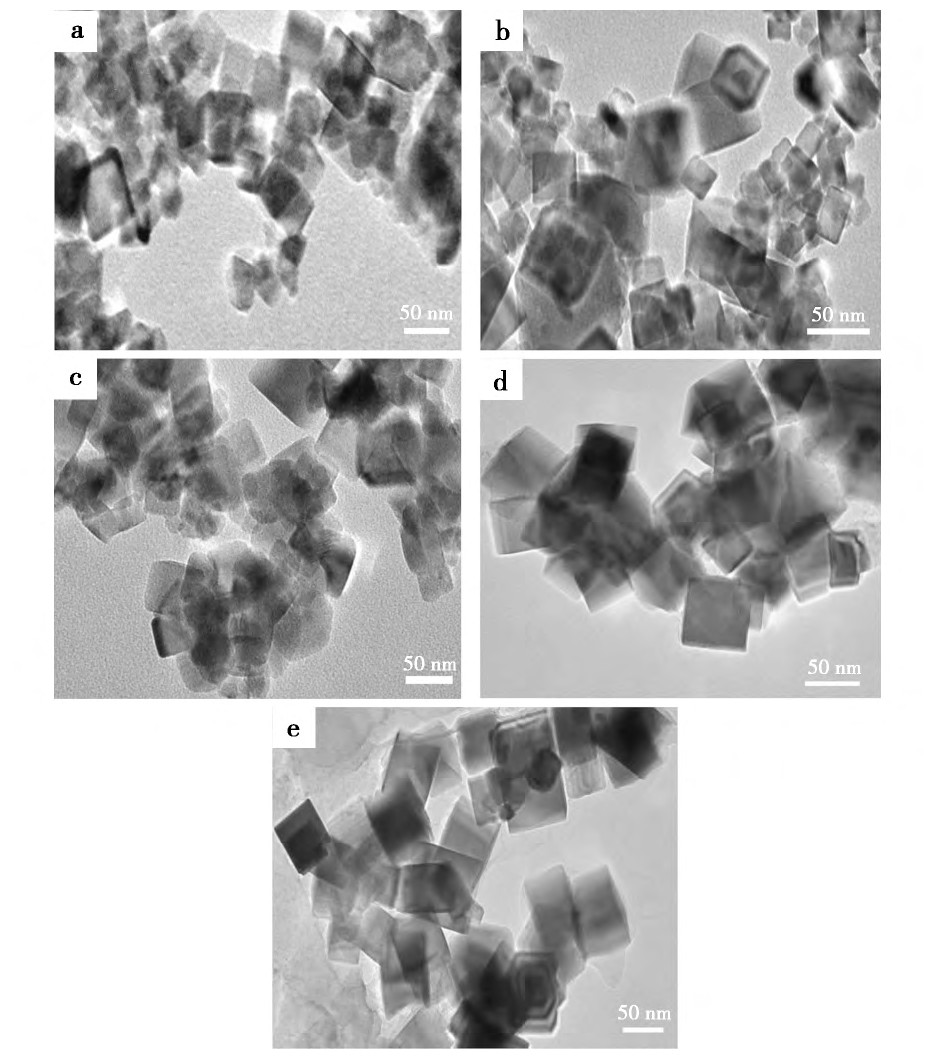
����ͼ2Ϊ��ͬˮ���¶Ⱥϳɵ�����Ce O2��TEMͼ����ͼ2���Կ�����ͬˮ���¶��ºϳɵ�����Ce O2��Ϊ�����塣����Nano Measurer�����õ�ˮ���¶�Ϊ100��120��140��160��180��ʱ, �ϳɵ���������Ce O2��ƽ�������ֱ�Ϊ29.6��32.54��39.21��43.02��50.38 nm�����������ˮ���¶ȵ�����, ����Ce O2����������������Ce O2������������Сȡ���ھ������ɵ��ٶȺ;��˳ɳ����ٶ�, Ȼ����������ʱ����Ҫ���¶ȱȾ��˳ɳ�ʱ����Ҫ���¶�Ҫ��, ����ڵ��������¾��˵������ٶȿ�, �����˳ɳ��ٶ���, �õ������ͽ�С���෴, ��ˮ���¶�����, ��������Һ����, ������, �����˾������������[17,18,19], �����������Ce O2��������ˮ���¶ȵ�����������
����ͼ2 ��ͬˮ���¶��ºϳɵ���������Ce O2��������羵ͼFig.2 TEM images of cubic nano-Ce O2synthesized at different hydrothermal temperatures
����a.100��;b.120��;c.140��;d.160��;e.180��
����2.2 Na OH��Ũ�ȶ�������Ӱ��
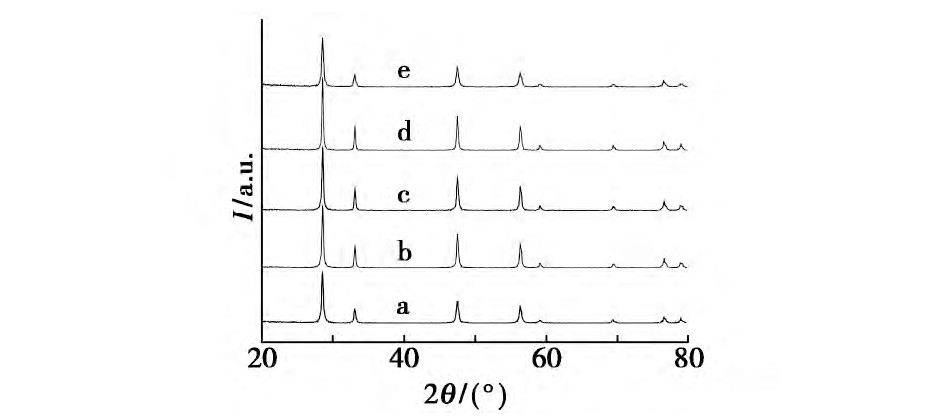
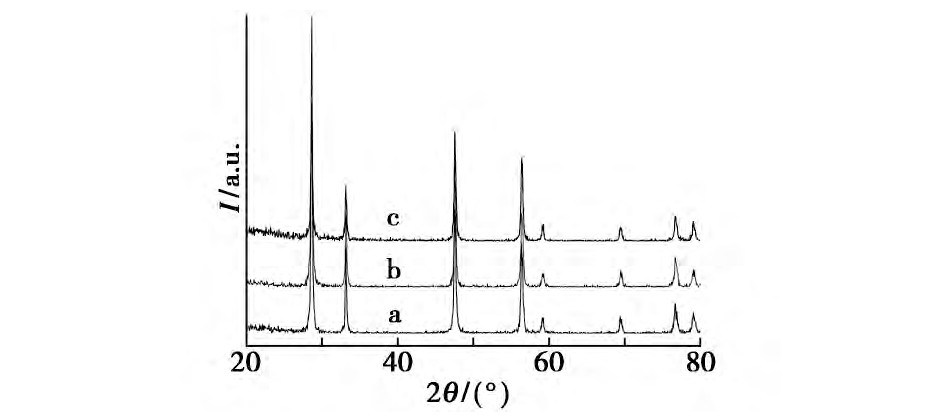
����������Ʒ���Ʊ�����, ���Һ�в����������Լ�, ���ı�Na OH��Ũ��, ̽�����������Ӱ��, �ֱ���������Һ��μ��뵽Ũ��Ϊ5.32��8.00��10.68��12.11��13.72 mol/L��Na OH��Һ��, �����������䡣ͼ3Ϊ��ͬŨ��Na OH��Һ�ºϳɵ�����Ce O2��XRDͼ����ͼ3���Կ���, ͨ���ı�Na OH��Ũ��, �ϳɵ�����Ce O2����������, ��û�����Եĸı䡣
����ͼ3 ��ͬŨ��Na OH�����ºϳɵ�����Ce O2������XRDͼFig.3 XRD patterns of cubic nano-Ce O2crystallites synthesized at different concentrations of Na OH solution
����a.5.32 mol/L;b.8.00 mol/L;c.10.68 mol/L;d.12.11 mol/L;e.13.72 mol/L
����ͼ4Ϊ��ͬŨ��Na OH�����ºϳɵ���������Ce O2������TEMͼ����ͼ4���Կ����ı�Na OH��Ũ�Ⱥϳɵ�����Ce O2������Ϊ�������ҷ�ɢ����ԽϺá�����Nano Measurer�����õ�Na OHŨ��Ϊ5.32��8.00��10.68��12.11��13.72 mol/Lʱ�ϳɵ���������Ce O2��ƽ�������ֱ�Ϊ32.26��36.20��55.26��38.89��27.30nm���ɴ˿��Կ�������Na OH��Ũ������, ����Ce O2�������ȳ����������, ��Na OH��Ũ�ȹ���ʱ, ������������������С��������Ϊ����Ӧ��Ũ�Ƚϵ�, �����ɾ��˵���������, ���˵������ٶȱȾ��������ٶ���, ���ŷ�Ӧ��Ũ�ȵ�������, �����������ٶȻ����ھ������ɵ��ٶ�, �γɿ����������ͻ����;����Ӧ��Ũ��̫��, ����Һ�л�˲�����ɴ����ľ���[20], ͬʱ�����������ٶȱ���, ����ڸ�Ũ�ȷ�Χ, ����������������Ũ�ȵ����Ӳ��ϼ�С��
����ͼ4 ��ͬŨ��Na OH�����ºϳɵ���������Ce O2������TEMͼFig.4 TEM images of cubic nano-Ce O2synthesized at different concentrations of Na OH solution
����a.5.32 mol/L;b.8.00 mol/L;c.10.68 mol/L;d.12.11 mol/L;e.13.72 mol/L
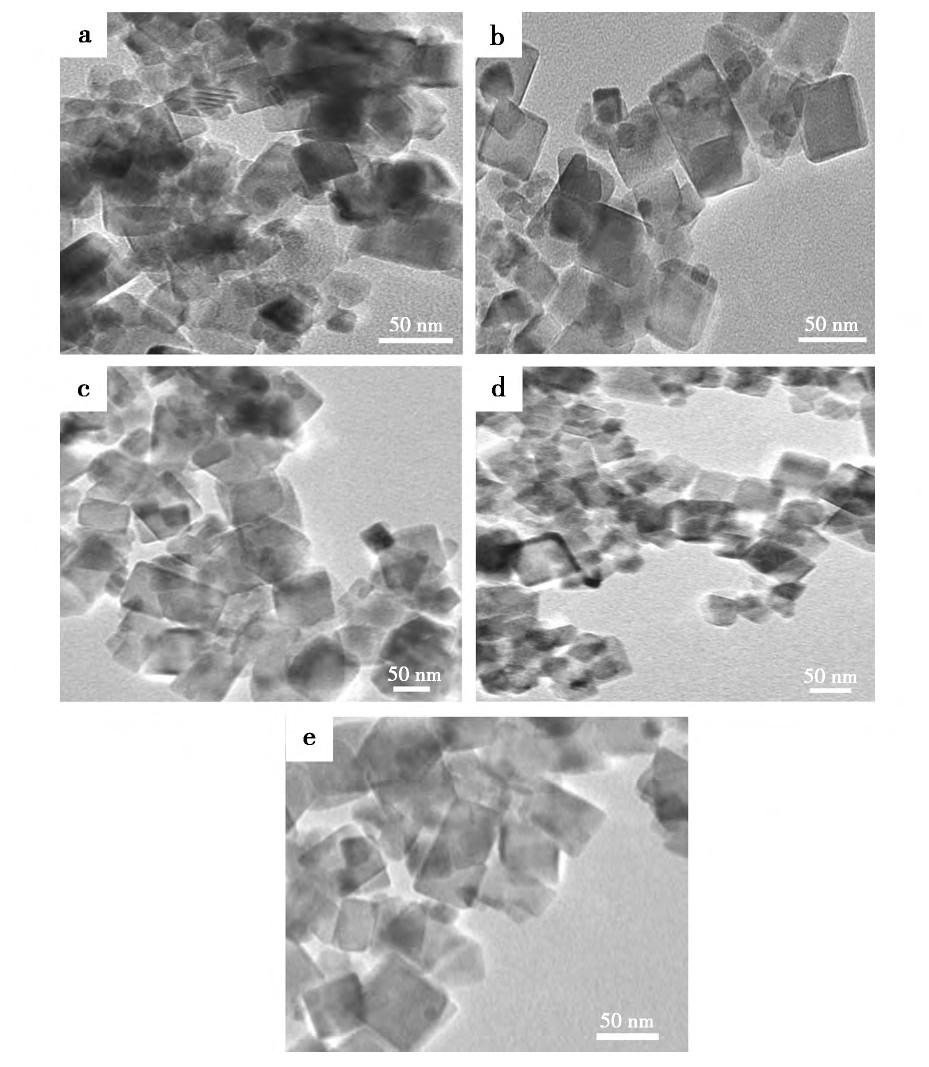
����2.3�� ��ͬ������Լ���������Ӱ��
����������Ʒ���Ʊ�����, �ֱ��ڻ��Һ�м�������ʵ����ľ��Ҷ���-2000���������ƺ;��Ҷ���-400, �����������䡣ͼ5Ϊ��ӦҺ�м��벻ͬ������Լ��ϳɵ�����Ce O2��XRDͼ����ͼ5�п��Կ���, ����3�ֱ�����Լ��ϳɵ�����Ce O2��������������, ���������ӷ塣��������������Լ���Ӱ����Ʒ�Ľᾧ�ȡ�
����ͼ5 ���벻ͬ������Լ��ϳɵ�����Ce O2��XRDͼFig.5 XRD patterns of cubic nano-Ce O2synthesized with different kinds of dispersants
����a.���Ҷ���-2000;b.��������;c.���Ҷ���-400
����ͼ6Ϊ���벻ͬ������Լ��ϳɵ�����Ce O2��TEMͼ����ͼ6���Կ������������Լ�������Ce O2�ķ�ɢ����һ���ĸ��ơ�����Nano Measurer�����õ�����ϵ�м�������ʵ����ľ��Ҷ���-2000���������ƺ;��Ҷ���-400�ϳɵ���������Ce O2��ƽ�������ֱ�Ϊ28.26��21.71��43.67 nm���ɴ˱������벻ͬ������Լ��������Ce O2������������ͬ��Ӱ�졣����ϵ��û�б�����Լ����, �ڷ�ӦҺ�м�����Ҷ���-2000���������ƺϳɵ�����Ce O2���������ż�С, ���Ǽ�����Ҷ���-400�ϳɵ�����Ce O2��������������ԭ�����ڷ�ӦҺ�м��������Լ���, ��������Ce O2���������γ�һ����ȵķ��Ӱ�����, �谭�˿���֮�����Ӵ�, �����˿���֮��ľ���, ʹ�ÿ����ĽӴ������ܡ�ͬʱ, ������Լ��ڹ�-Һ�������γ�һ�����ܵ�˫��㱣��Ĥ, �Ӷ��谭��С�����ľۼ�[21]��
����ͼ6 ���벻ͬ������Լ��ϳɵ�����Ce O2��TEMͼFig.6 TEM images of cubic nano-Ce O2 synthesized with different kinds of dispersants
����a.���Ҷ���-2000;b.��������;c.���Ҷ���-400
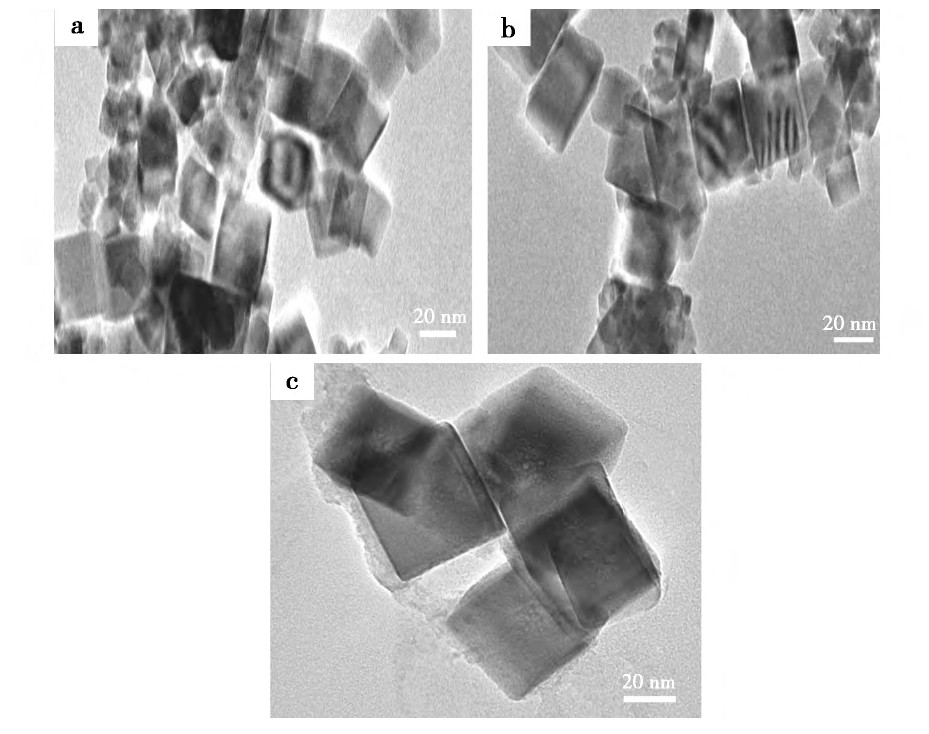
�������������������ӱ�����Լ�, ������Ce O2ǰ���������, �γɵ�˫��㱣��Ĥ�ľ���������ô�, ʹ�ÿ�����ʹ��������ײ, Ҳ��ֿ�, �Ӷ���Ч����ֹ�˿����Ķ����ž�, ����Ʊ�������Ce O2���������С�����Ҷ���-2000�;��Ҷ���-400Ϊ�����ӱ�����Լ�, ������Ce O2ǰ����������γ�һ����ȵķ��Ӱ�����, �������Ҷ����ij����ṹ�����ռ�λ��ЧӦ, ������Խ�������Ŀռ�λ��Խ����ϵ�м�����ͬŨ�ȵľ��Ҷ���-2000�;��Ҷ���-400ʱ, ���Ҷ���-2000�ϳɵ�����Ce O2��������С�����Ҷ���-400�ķ�������Խ϶�, ������Ce O2ǰ������������Ŀռ�λ��С, ���������֮��ij���С, ��������������ž�, ���ҿ�������Ϊ����ľ��Ҷ���-400Ũ��ƫ��, ���ӱ���ֻ�������ľ��Ҷ���-400�����������Ҳ��������������ž�, ��˵õ�������Ce O2�����������
����2.4�� �����¶ȶ�������Ӱ��
����������Ʒ���Ʊ�����, ���Һ�в����������Լ�, ���ı������¶�, ̽�����������Ӱ��, ����������Ʒ�ֱ���400��600��700���±���2 h, �����������䡣ͼ7Ϊ��ͬ�����¶Ⱥϳɵ�����Ce O2��XRDͼ����ͼ7��֪, ��ͬ�����¶Ⱥϳɵ�����Ce O2������������������ӷ�����������
����ͼ7 ��ͬ�����¶��ºϳ�����Ce O2��XRDͼFig.7 XRD patterns of cubic nano-Ce O2synthesized at different calcination temperatures
����a.400��;b.600��;c.700��
����ͼ8Ϊ��ͬ�����¶Ⱥϳɵ�����Ce O2��TEMͼ, ��ͼ��֪, �ı������¶Ⱥϳɵ�����Ce O2��Ϊ�������������¶ȶ�����Ce O2��������òӰ�첻������Nano Measurer�����õ������¶�Ϊ400��600��700��ϳɵ�����Ce O2��ƽ�������ֱ�Ϊ50.75��49.61��52.40 nm��������ڲ�ͬ�����¶��ºϳɵ�����Ce O2����������, ��ԭ�������չ���ǰ�����ʧȥ������ˮ, �������ĹǼܲ�û�з����仯��
����ͼ8 ��ͬ�����¶��ºϳ�����Ce O2��TEMͼFig.8 TEM images of cubic nano-Ce O2synthesized at different calcination temperatures
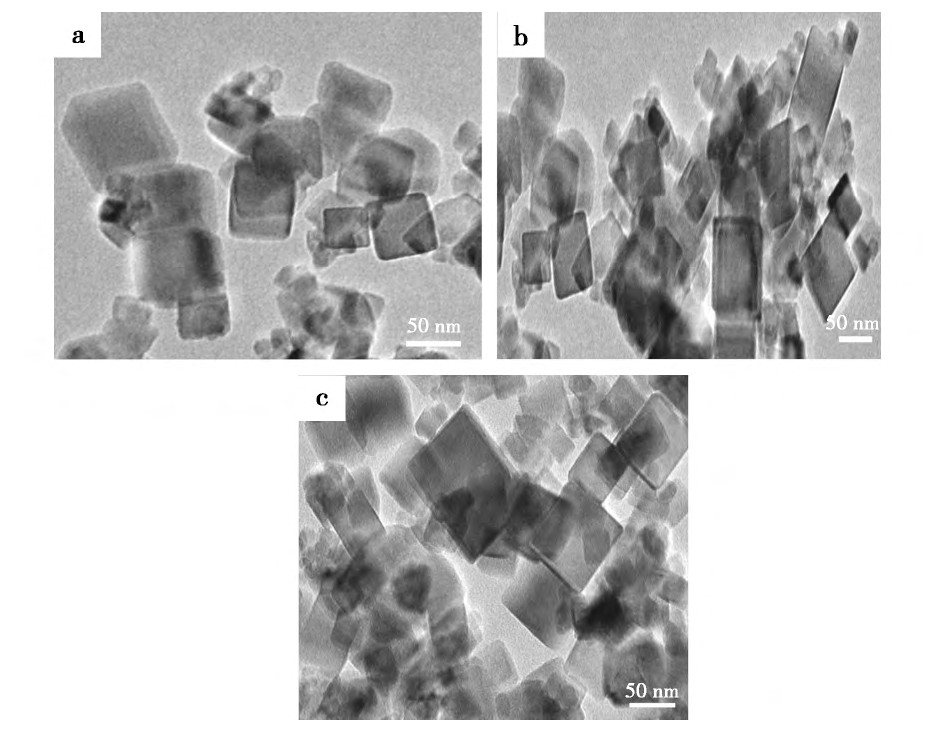
����a.400��;b.600��;c.700��
����3�� ����
����3.1�� �Ʊ�������ˮ���¶ȡ�Na OH��Ũ�Ⱥͱ�����Լ���������Ӱ����������Ҫ����, ����ˮ���¶ȵ�����, ����������;����Na OHŨ�ȵ�����, ������������С;���벻ͬ�ı�����Լ�������������ͬ��Ӱ��, ������Ҷ���-2000����������ʹ����Ce O2���������Ա�С, ��������Ҷ���-400ʹ����Ce O2���������Ա��
����3.2�� XRDͼ��TEMͼ��������ˮ�ȷ��ܹ��ɿغϳɲ�ͬ�������ᾧ�ȸߵ���������Ce O2������
�����ο����ף�
����[1]DIMITRATOS N, LOPEZ-SANCHEZ J A, HUTCHINGS G J.Chem Inform abstract:selective liquid phase oxidation with supported metal nanoparticles[J].Cheminform, 2012, 43 (13) :20-44.
����[2]�����, �����, ����, ��.�ض���ò�Ͷ������Ce O2���Ʊ�����CO�������о���չ[J].������չ, 2017, 36 (7) :2 481-2 487.
����[3]HE Yong-jun.Nanostructured Ce O2microspheres synthesized by a novel surfactant-free emulsion[J].Powder.Technol., 2005, 155 (1) :1-4.
����[4]HANEDA M, KANEKO T, KAMIUCHI N, et al.Improved three-way catalytic activity of bimetallic Ir-Rh catalysts supported on Ce O2-Zr O2[J].Catal.Sci.Technol., 2015, 5 (3) :1 792-1 800.
����[5]л��, �, ����ƽ, ��.��������/����ˮ��ʯ/����̿���Ʊ�������Ӧ��[J].������չ, 2016, 35 (1) :182-188.
����[6]����, ��־��, ��ǿ, ��.����ɢ����Ce O2��Cu-Ce-O�������Ʊ����������������[J].���ܲ���, 2009, 40 (2) :311-313.
����[7]��, ����.��ϸ������ᾧ�Ⱥ������Կ���������Ӱ���о�[J].���������ѧѧ�� (��Ȼ��ѧ��) , 2012, 38 (1) :106-112.
����[8]����.��������������Ʊ��������ܵ��о�[D].�Ͼ�:�Ͼ�������ѧ, 2005.
����[9]NATILE M M, GLISENTI A.Nanostructured oxide-based powders:investigation of the growth mode of the Ce O2clusters on the YSZ surface[J].J.Phys.Chem.B, 2006, 110 (6) :2 515-2 521.
����[10]LI Ling, YANG H K, MOON B K, et al.Photoluminescence properties of Ce O2��Eu3+nanoparticles synthesized by a sol-gel method[J].J.Phys.Chem.C, 2009, 113 (2) :610-617.
����[11]HE Yong-jun, YANG Bo-lun, CHENG Guang-xu.Controlled synthesis of Ce O2nanoparticles from the coupling route of homogenous precipitation with microemulsion[J].Mater.Lett., 2003, 57 (13/14) :1 880-1 884.
����[12]LIU Bo, YAO Ming-guang, LIU Bing-bing, et al.Highpressure studies on Ce O2nano-octahedrons with a (111) -terminated surface[J].J.Phys.Chem.C, 2011, 115 (11) :4 546-4 551
����[13]ZHANG Zhi-xin, WANG Ye-hong, WANG Min, et al.An investigation of the effects of Ce O2, crystal planes on the aerobic oxidative synthesis of imines from alcohols and amines[J].Chin.J.Catal., 2015, 36 (9) :1 623-1 630.
����[14]CHU Xi, CHUNG W L, LANNY D S.Sintering of sol-gelprepared submicrometer particles studied by transmission electron microscopy[J].J.Am.Ceram.Soc., 1993, 76 (8) :2 115-2 118.
����[15]������, ������, �Ž���, ��.������������Ʊ���Ӧ���о���[J].Ӧ�û���, 2014, 24 (9) :1 701-1 704.
����[16]������, ����¶, ����, ��.ˮ�ȷ��Ʊ�������������漰��CO�����������о�[J].�������ͨѶ, 2017, 43 (4) :146-147.
����[17]���Ź�, ��Ľ�, ����, ��.������������Ʊ����������[J].������չ, 2014, 33 (3) :720-723.
����[18]GUO Ming, DIAO Peng, WANG Xin-dong, et al.The effect of hydrothermal growth temperature on preparation and photoelectrochemical performance of Zn O nanorod arrays[J].J.Solid State Chem., 2005, 178 (10) :3 210-3 215.
����[19]WANG Yu-juan, JIE Xue-fei, DONG Xin-fa, et al.Function of ceria in oxidation catalysis[J].Chin.J.Power.Sources., 2002, 26 (1) :43-46.
����[20]���Ĺ�, ��ͬ��, ��С��, ��.��ͬ��òFe4O3�������ӵ������������Ʊ������[J].����ѧѧ��, 2006, 22 (7) :1 263-1 268.
����[21]����, ʯ����.������Լ�����������п��������ò��Ӱ���о�[J].�������Ͳ���, 2007, 35 (8) :43-44.





