����ժ Ҫ����Ŀ�� �о���Ҷ�ܽ�Patrinia heterophylla Bunge�Ļ�ѧ�ɷּ���������ԡ����� ��Ҷ�ܽ�75%�Ҵ���ȡ����ô����֬���轺����ѹҺ�ࡢ���Ʊ���ѹҺ����з��봿��, �����������ʼ��������ݼ������û�����Ľṹ��CCK-8���������������ư�Ѫ��ϸ��HL-60��K562���ԡ���� ���з���õ�7��������, �ֱ����Ϊpatrinoside (1) ��roseoside�� (2) �� (3-O-α-L-arabinopyranosyl hederagenin 28-O-β-D-glucopyranosyl- (1→6) -β-D-glucopyranoside (3) ��apigenin4′-O-β-D-glucopyranoside (4) ��fissoside B (5) ���۲���-7-O-β-D-«������ (6) ��rhamnocitrin 3-O-[α-L-rhamnopyranosyl (l→4) -O-α-L-rhamnopyranosyl (1→6) ]-β-D-galactopyranoside (7) ��������1��6��IC50<100μg/mL������ ������2��6Ϊ�״δӸ���ֲ���з���õ�, ������1Ϊ�״δӸ�ֲ���з���õ���������1��3��5��6��HL-60ϸ������һ���Ķ�����������, ������2��6��K562ϸ������һ���Ķ����������á�
�����ؼ��ʣ�����Ҷ�ܽ�; ��ѧ�ɷ�; �������; �������;
����Abstract����AIM To study the chemical constituents from Patrinia heterophylla Bunge. and their biological activities.METHODS The 75% ethanol extract from P. heterophylla was isolated and purified by macroporous resin, silica, medium pressureand liquid phase and semi-preparative HPLC, then the structures of obtained compounds were identified by physicochemical properties and spectral data. The activities of inhibiting leukemia cells HL-60 and K562 were detected by CCK-8 method. RESULTS Seven compounds were isolated and identified as patrinoside (1) ��roseoside �� (2) �� (3-O-α-L-arabinopyranosyl hederagenin 28-O-β-D-glucopyranosyl- (1→6) -β-D-glucopyranoside (3) ��apigenin4′-O-β-D-glucopyranoside (4) ��fissoside B (5) ��apigenin-7-O-β-D-lutinoside (6) ��rhamnocitrin 3-O-[α-L-rhamnopyranosyl (l→4) -O-α-L-rhamnopyranosyl (1→6) ]-β-D-galactopyranoside (7) . IC50 values of compounds 1-6 were below 100 μg/mL.CONCLUSION Compounds 2-6 are isolated from genus Patrinia for the first time, and compound 1 is isolated from this plant for the first time. Compounds 1-3 and 5-6 have a certain toxicity inhibition effects on HL-60 cells, while compounds 2-6 have a certain toxic inhibitory effects on K562 cells.
����Keyword����Patrinia heterophylla Bunge; chemical constituents; isolation and identification; biological activities;
������Ҷ�ܽ�Patrinia heterophylla Bunge�ǰܽ��ưܽ����������ݱ�ֲ��, �����������Ҷ�ܽ��ĸ����������, ҩ����Ĺͷ��[1]�����ױ���Ĺͷ�ضԸ��༱����Ѫ��ϸ������������, �����ٴ����������ư�Ѫ��[2-3]����Ҷ�ܽ���ΪĹͷ�ص�ҩԴֲ��, ���仯ѧ�ɷֱ�������, Ϊ��һ����ȷ��Ҷ�ܽ��е�ҩЧ���ʻ���, ��ʵ�����Ҷ�ܽ����л�ѧ�ɷ��о�, �����䵥�廯������п���������ɸѡ�����з���õ�7��������, ������2��6��Ϊ�״δӸ���ֲ���з���õ�, ������1Ϊ�״δӸ�ֲ���з���õ�, ������1��6��Ѫ��ϸ����һ�����������á�
����1�� ���������
������ЧҺ��ɫ���� (�ձ� Hitachi��˾) ;2000ES������ɢ������ (����Altech��˾) ;R1-102ʾ������ (�ձ�Shodex��˾) ;NP7000���Ʊ�Һ��� (���˾) ;R200��ת�����ǡ���ѹ����ɫ���� (��ʿBuchi��˾) ;Bruker AV-�� HD 600�����˴Ź����� (��ʿBruker��˾) ;Q-TOF�߷ֱ������� (����Sciex��˾) ; YT-CJ-1ND ��������̨ (������̩��¡��������˾) ;MCO-15ACCO2 ���������� (�ձ�Sanyo��˾) ;DT5-2���Ļ� (����ʱ���������Ļ�����˾) ;1420-012ø���� (����Perkin Elmer��˾) ;ECLIPSE TS100���� (�ձ�Nikon��˾) ;D-101�����֬ (����Ͽ��ͳɿƼ�����˾) ;��������轺GF254���������轺100��200Ŀ (�ൺ������) ��������ʯ���ѡ��ȷ¡���ͪ�������������������ͼ״����Լ� (��ҩ���Ż�ѧ�Լ�����˾) ;ɫ�״��״������� (��ҩ���Ż�ѧ�Լ�����˾) ;��������ˮ (����������˾) ��RPMI 1640 ������ (����Gibco��˾) ;̥ţѪ�� (����HyClone��˾) ;�������� (����Amresco��˾) ;CCK-8�Լ��� (Biosharp����Ƽ���˾) ��

������Ҷ�ܽ���2014����ں���ʡ, ���й������ž���ҽԺ��Ƽ�о�Ա����Ϊ��Ʒ��
��������������Ѫ��ϸ�� (HL-60) ����������ϸ����Ѫ��ϸ�� (K562) �������й�ҽѧ��ѧԺ����ҽԺϸ�����ġ�
����2�� ��ȡ�����
�����������Ҷ�ܽ�ȫ�� (2 kg) ����, 75%�Ҵ�������ȡ3��, ���˺��ѹŨ������Ҷ�ܽ��ܽ��ࡣ�ܽ����ˮ������Һ, ������ʯ���ѡ��ȷ¡���������������������ȡ3��, ȡ�л����, ����ת�����Ǽ�ѹŨ��, �����ܼ�, �ֱ��ʯ���ѡ��ȷ¡�������������������λ63��112��36��95 g��ȡ�����������ô����֬ɫ���з���, ������30%��60%��95%�Ҵ������ݶ�ϴ��, ��ѹŨ����õ�30%��60%��95%���ֽ��ࡣ
������30%���ֽ��� (50 g) ���轺�� (100��200 Ŀ) ����, ���ȷ�-�״�-ˮ (25��1��0��9��1��0��9��1��0.1��8��2��0.2��7��3��0.5) �ݶ�ϴ��, ���õ�263�����A1-263, ͨ������ɫ���, �ϲ���ͬ��֡����A180-190�ϲ��轺��, ���ȷ�-�״�-ˮ (9��1��0.1��8��2��0.2) �ݶ�ϴ��, �پ����Ʊ�HPLC (����-ˮ 2��8) �û�����1 (21.5 mg) �����A120-159�ϲ�����ѹ������������-ˮ (1��9��3��17��1��4��1��3��3��7) �ݶ�ϴ��, �û�����2 (12.1 mg) ��
������60%���ֵĽ��ྭ�轺��ɫ�� (100��200 Ŀ) ����, ���ȷ�-�״�-ˮ (9��1��0.1��8��2��0.2��7��3��0.5) �ݶ�ϴ��, ���õ�245�����B1-245, ͨ������ɫ���, �ϲ���ͬ��֡����B93-100�ϲ�����ѹ�������Լ״�-ˮ (5��95��55��45) �ݶ�ϴ�ѵû�����3 (11 mg) �����B60-70�źϲ�����ѹ������������-ˮ (3��97��48��52) �ݶ�ϴ��, F23�ؽᾧ�û�����4 (16.4 mg) , F26�ؽᾧ�û�����5 (9.1 mg) �����B77-91�źϲ�����ѹ�������Լ״�-ˮ (5��95��80��20) �ݶ�ϴ��, F30�ؽᾧ�û�����6 (8.8 mg) , F40�ؽᾧ�û�����7 (10.6 mg) ��
����3�� �ṹ����
����������1:��ɫ��ĩ (�״�) ��ESI-MS m/z:485[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 1.78 (1H, m, H-13) , 2.03 (2H, m, H-6) , 2.13 (1H, m, H-9) , 2.19 (2H, dd, J=7.2 Hz, H-12) , 2.95 (1H, q, J=7.8 Hz, H-8) , 3.15 (1H, t, J=8.4 Hz, H-7) , 3.24 (2H, m, H-10) , 4.04 (1H, d, J=11.4 Hz, H-1) , 4.22 (1H, m, H-11) ; 13C-NMR (150 MHz, CD3OD) δ: 93.5 (C-1) , 140.0 (C-3) , 116.4 (C-4) , 34.1 (C-5) , 40.9 (C-6) , 73.3 (C-7) , 49.0 (C-8) , 42.7 (C-9) , 62.2 (C-10) , 69.6 (C-11) , 103.4 (C-1′) , 75.1 (C-2′) , 78.1 (C-3′) , 71.7 (C-4′) , 77.9 (C-5′) , 62.8 (C-6′) , 173.3 (C-1″) , 44.1 (C-2″) , 26.8 (3″) , 22.6 (4″) ����������������[4]һ��, �ʼ���Ϊpatrinoside��
����������2:��ɫ��ĩ (�״�) ��ESI-MS m/z:677[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 2.10 (1H, d, J=16.8 Hz, H-2) , 2.47 (1H, d, J=16.8 Hz, H-2) , 5.81 (1H, quin, H-4) , 4.36 (1H, m, H-9) , 1.21 (3H, d, J=6.6 Hz, H-10) , 0.90 (3H, s, H-11) , 0.91 (3H, s, H-12) , 1.86 (3H, d, J=1.2 Hz, H-13) , 4.28 (3H, d, J=7.8 Hz, Glu-1′) ;13C-NMR (150 MHz, CD3OD) δ: 42.4 (C-1) , 50.6 (C-2) , 201.1 (C-3) , 127.1 (C-4) , 167.2 (C-5) , 79.9 (C-6) , 131.5 (C-7) , 135.2 (C-8) , 77.2 (C-9) , 21.1 (C-10) , 23.4 (C-11) , 24.6 (C-12) , 19.5 (C-13) , 102.7 (C-1′) , 75.2 (C-2′) , 78.1 (C-3′) , 71.6 (C-4′) , 77.9 (C-5′) , 62.8 (C-6′) ����������������[5]һ��, �ʼ���Ϊroseoside ��
����������3:��ɫ��ĩ (�״�) ��ESI-MS m/z:951[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 3.06 (1H, dd, J=4.8 Hz, H-3) , 5.20 (1H, brs, H-12) , 4.21 (1H, d, J=8.4 Hz, H-Ara-1) , 4.29 (1H, d, J=8.4 Hz, H-Glu-1′) , 5.30 (1H, d, J=7.8 Hz, H-Glu-1) ; 13C-NMR (150 MHz, CD3OD) δ: 39.8 (C-1) , 27.0 (C-2) , 90.7 (C-3) , 40.1 (C-4) , 57.0 (C-5) , 19.3 (C-6) , 33.9 (C-7) , 40.7 (C-8) , 49.0 (C-9) , 37.9 (C-10) , 24.5 (C-11) , 123.8 (C-12) , 144.8 (C-13) , 42.8 (C-14) , 28.9 (C-15) , 24.0 (C-16) , 49.0 (C-17) , 41.8 (C-18) , 41.4 (C-19) , 36.7 (C-20) , 29.2 (C-21) , 32.4 (C-22) , 28.5 (C-23) , 16.9 (C-24) , 16.1 (C-25) , 17.8 (C-26) , 26.3 (C-27) , 178.0 (C-28) , 74.3 (C-29) , 107.1 (C-Ara-1) , 78.1 (C-Ara-2) , 72.8 (C-Ara-3) , 69.5 (C-Ara-4) , 66.3 (C-Ara-5) , 95.7 (C-Glu-1) , 73.8 (C-Glu-2) , 78.0 (C-Glu-3) , 70.9 (C-Glu-4) , 78.0 (C-Glu-5) , 69.5 (C-Glu-6) , 104.6 (C-Glu-1′) , 75.4 (C-Glu-2′) , 78.0 (C-Glu-3′) , 71.5 (C-Glu-4′) , 77.8 (C-Glu-5′) , 62.7 (C-Glu-6′) ����������������[6]һ��, �ʼ���Ϊ (3-O-α-L-arabinopyranosyl hederagenin 28-O-β-D-glucopyranosyl- (1→6) -β-D-glucopyranoside��
����������4: ��ɫ���� (DMSO) ��ESI-MS m/z:455[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 7.95 (2H, d, J=8.4 Hz, H-2′, 6′) , 6.93 (2H, d, J=8.4 Hz, H-3′, 5′) , 6.82 (1H, s, H-3) , 5.06 (1H, d, J=7.8 Hz, H-Glu-1) , 3.76 (1H, s, H-Glu-6) ; 13C-NMR (150 MHz, CD3OD) δ: 182.0 (C-4) , 164.2 (C-7) , 162.9 (C-2) , 161.4 (C-4′) , 161.1 (C-9) , 156.9 (C-5) , 128.6 (C-2′, C-6′) , 121.0 (C-1′) , 116.0 (C-3′, C-5′) , 105.3 (C-10) , 103.1 (C-3) , 99.9 (C-Glu-1) , 99.5 (C-6) , 94.8 (C-8) , 77.2 (C-Glu-3) , 76.4 (C-Glu-5) , 73.1 (C-Glu-2) , 69.5 (C-Glu-4) , 60.6 (C-Glu-6) ����������������[7]һ��, �ʼ���Ϊ apigenin4′-O-β-D-glucopyranoside��
����������5:��ɫ�뾧 (DMSO) ��ESI-MS m/z:483[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 5.00 (1H, m, H-10) , 4.78 (1H, s, H-10) , 4.58 (1H, s, H-1″) , 4.35 (1H, d, J=7.8 Hz, H-3) , 4.23 (1H, d, J=7.8 Hz, H-1′) , 2.42 (1H, t, J=5.4 Hz, H-1) , 2.28 (1H, m, H-7) , 1.63 (1H, d, J=9.6 Hz, H-7) , 2.12 (1H, m, H-4) , 1.95 (1H, overlap, H-4) , 1.23 (3H, s, H-8) , 0.61 (3H, s, H-9) ; 13C-NMR (150 MHz, CD3OD) δ: 150.8 (C-2) , 113.4 (C-10) , 101.1 (C-1′) , 100.9 (C-1″) , 76.6 (C-3′) , 75.2 (C-5′) , 73.3 (C-2′) , 71.8 (C-3) , 70.7 (C-4′) , 70.5 (C-3″) , 70.3 (2″) , 68.3 (C-5″) , 67.2 (C-6′) , 50.0 (C-1) , 40.3 (C-6) , 39.9 (C-5) , 31.9 (C-4) , 26.8 (C-7) , 25.8 (C-8) , 21.9 (C-9) , 17.9 (C-6″) ����������������[8]һ��, �ʼ���Ϊfissoside B��
����������6:��ɫ���� (DMSO) ��ESI-MS m/z:577[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 1.07 (3H, d, J=6.0 Hz, H-6?) , 4.54 (1H, d, J=6.0 Hz, H-1?) , 5.06 (1H, d, J=7.2 Hz, H-1″) , 6.44 (1H, s, H-6) , 6.76 (1H, s, H-8) , 6.85 (1H, s, H-3) , 6.95 (2H, d, J=8.4 Hz, H-3′, H-5′) , 7.95 (2H, d, J=8.4 Hz, H-2′, H-6′) , 10.37 (1H, s, 4′-OH) , 12.95 (1H, s, 5′-OH) ;13C-NMR (150 MHz, CD3OD) δ: 17.8 (C-6?) , 66.0 (C-6″) , 68.3 (C-5?) , 69.5 (C-2?) , 70.3 (C-3?) , 70.7 (C-4″) , 72.0 (C-2″) , 73.1 (C-4?) , 75.6 (C-3″) , 76.3 (C-5″) , 94.8 (C-8) , 99.5 (C-6) , 99.9 (C-1?) , 100.5 (C-1″) , 103.1 (C-3) , 105.4 (C-10) , 116.1 (C-3′) , 121.0 (C-1′) , 128.6 (C-2′, C-6′) , 156.9 (C-9) , 161.2 (C-4′) , 161.3 (C-7) , 162.9 (C-5) , 164.4 (C-2) , 182.0 (C-4) ����������������[9]һ��, �ʼ���Ϊ�۲���-7-O-β-D-«�����ա�
����������7:��ɫ���� (�״�) ��ESI-MS m/z:807[M+Na]+��1H-NMR (600 MHz, CD3OD) δ: 3.83 (3H, s, H-7) , 5.01 (1H, d, J=7.8 Hz, H-1″) , 6.29 (1H, d, J=7.2 Hz, H-6) , 6.55 (1H, s, H-8) , 6.83 (2H, d, J=9.0 Hz, H-3′, H-5′) , 8.07 (2H, d, J=8.4 Hz, H-2′, H-6′) ;13C-NMR (150 MHz, CD3OD) δ: 179.7 (C-4) , 167.4 (C-7) , 162.7 (C-5) , 161.8 (C-4′) , 159.7 (C-9) , 158.5 (C-2) , 135.9 (C-3) , 132.6 (C-2′, C-6′) , 122.5 (C-1′) , 116.2 (C-3′) , 106.5 (C-10) , 105.5 (C-1″) , 104.0 (C-1″″) , 101.9 (C-1?) , 99.2 (C-6) , 93.3 (C-8) , 79.6 (C-4?) , 75.4 (C-5″) , 75.0 (C-3″) , 74.0 (C-4″″) , 73.2 (C-3″″) , 73.0 (C-2″″) , 72.2 (C-2″) , 72.1 (C-3?) , 71.9 (C-2?) , 70.2 (C-5″″) , 70.0 (C-5?, C-4″) , 67.6 (C-6″) , 56.6 (C-OMe) , 18.0 (C-6″″) , 18.0 (C-6?) ����������������[10]һ��, �ʼ���Ϊrhamnocitrin 3-O-[α-L-rhamnopyranosyl (l→4) -O-α-L-rhamnopyranosyl (1→6) ]-β-D-galactopyranoside��
����4�� ���Բ���
����4.1�� ϸ������
������ϸ����Һ������ȡ��, ���淽��[11-12]����, ��ϸ���ú�15%̥ţѪ���RPMI 1640����Һ, ��37 ��, 5%CO2 ����ʪ�����������������ݲ�ͬϸ���������ٶ�1��2 d�������ʵ�����Һ���ߴ�����
����4.2�� CCK-8���
���������ڶ��������ڵ�HL-60��K562ϸ���Ƴ��ܶ�9×104/mL��8×104/mL��ϸ����Һ������96�װ���, ÿ��100 μL, ÿ������5��ƽ�пס���������������24 h��ֱ���벻ͬ����Ũ�ȵĻ�����1��6 (�ӵ͵�������Ϊ0��20��40��60��80��100 μg/mL) �����Զ���Ʒ˳��50 μL����ҩ�������������������48 h, ȡ��������, ÿ����10 μL CCK-8 ��Һ��1��2 h (K562 1.5 h, HL-60 2 h) ����450 nm����ODֵ��������ϸ�������ʼ�IC50ֵ��
����4.3�� ��Ѫ��ϸ����ֳ���ƻ��Բⶨ
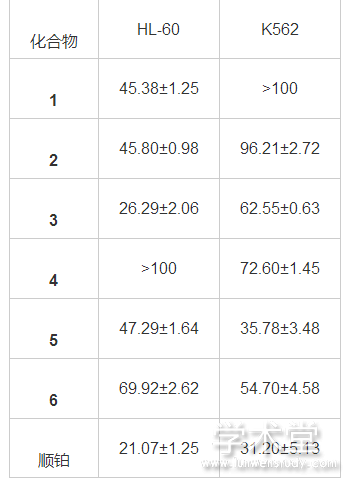
�������������IC50ֵ����1����������ױ���[13], ���廯�����IC50<100 μg/mL, ����ʵ����Ʒ�������Ե�ϸ�������ԡ������ʾ, ������1��3��5��6��HL-60ϸ������һ���Ķ�����������, ������2��6��K562ϸ������һ���Ķ����������á�
����5�� ����
������Ҷ�ܽ���Ĺͷ�������ҩԴֲ��[14], ��������������Ĺͷ��ʹ�õ�����, ���ǹ�����Ҷ�ܽ��Ļ�ѧ�ɷֱ���ȴ����, Ŀǰ��������12�������ࡢ5����ϩ��������3��ľ֬�ء�2�������ࡢ3����ͪ�ࡢ1�����Ǻ�1���㶹�������ı�������Ҷ�ܽ������պʹ��Ƽ����������Ƽ�����Ѫ�����ӹ������������ʹ�[15], �����䷢��ҩЧ��ȷ�л�������δȷ�������о�����Ҷ�ܽ��з���õ�7��������, ���л�����1Ϊ��ϩ�����ࡢ������2Ϊ���������������ࡢ������3Ϊ���������ࡢ������5Ϊ�����������ա�������4��6��7Ϊ��ͪ�ࡣͨ��CCK-8��ɸѡ�˻�����1��6��HL-60��K562ϸ������ֳ�������á������ʾ, �����廯�����Ѫ��ϸ������һ���Ķ�����������, �һ�����1��3��5��6��HL-60ϸ����������2��6��K562ϸ��������������ҩ������Ũ����һ����������ϵ, ����Ϊ����һ���о���Ҷ�ܽ��Ŀ���Ѫ�������ṩ�ο�����Ȼ���廯�����Ѫ��ϸ�����ֳ������ⶾ��, ����ҩ�ɷָ���, ��Ҷ�ܽ���ѧ�ɷֿ���Ѫ�����õ�ȷ�л��ƻ����һ���о���
������1 ���������Ѫ��ϸ��IC50ֵ (μg/mL)
���������
����[1] �»���, �Ͼ���.�����ܽ���ҩ��ֲ����������[J].�й���ҩ��־, 1994, 19 (2) :67-70.
����[2] ������, ���Ļ�, ʷ����, ��.Ĺͷ�ض�HL-60ϸ���յ��ֻ����õ�ʵ���о�[J].�й���ҽҩ�Ƽ�, 1999, 6 (6) :415.
����[3] ������, ����.Ĺͷ����ȡ���յ�K562ϸ��������ʵ���о�[J].������ҽҩ��ѧѧ��, 2007, 30 (1) :51-53.
����[4] Nishiya K, Kimura T, Takeya K, et al.Sesquiterpenoids and iridoid glycosides from Valeriana fauriei[J].Phytochemistry, 1992, 31 (10) :3511-3514.
����[5] Otsuka H, Yao M, Kamada K, et al.Alangionosides G-M:glycosides of megastigmane derivatives from the leaves of Alangium premnifolium[J].Chem Pharm Bull (Tokyo) , 1995, 43 (5) :754-759.
����[6] Haruhisa K, Satoshi H, Mizumi S, et al.Studies on the constituents of Hedera rhombea BEAN.��.on the hederagenin glycosides. (2) [J].Chem Pharm Bull, 1985, 33:3473-3478.
����[7] Teng R W, Xie H Y, Li H Z, et al.Two new acylated flavonoid glycosides from Morina nepalensis var.alba Hand.-Mazz[J].Magn Reson Chem, 2002, 40:415-420.
����[8] Wang M, Zhang Y, Zhang H, et al.The active glycosides from Urtica fissa rhizome decoction[J].J Nat Med, 2018, 72 (2) :557-562.
����[9] ����, ��ϸ��, ������.��ҩ�ؽ����۲������������[J].ҩѧѧ��, 2009, 44 (5) :496-499.
����[10] Lin C N, Chung M I, Gan K H, et al.Flavonol and anthraquinone glycosides from Rhamnus formosana[J].Phytochemistry, 1991, 30 (9) :3103-3106.
����[11] �ױ�, ������, �����, ��.ѻ�����Ͷ��˸ΰ�ϸ����ֳ���������ü�����[J].�ִ�����ҽѧ, 2016, 24 (8) :1177-1181.
����[12] Freshney R I.Culture of animal cells:A manual of basictechnique and specialized applications, sixth edition[M].Hoboken:John Wiley & Sons, Inc., 2011.
����[13] ֣��, ��ʤ��, �ܻ�, ��.�{�黨���Ļ�ѧ�ɷּ���������о�[J].�в�ҩ, 2015, 46 (2) :189-193.
����[14] ������, ʯ����.Ĺͷ�صı��ݿ�֤[J].��ҽҩѧ��, 2011, 39 (2) :120-122.
����[15] �½���, �����.��Ҷ�ܽ���߰Ƭ���������õ�ʵ���о�[J].��ҽ�о�, 2006, 19 (2) :17-19.





